Botany – Water in
Plants & Plant Nutrients
I. Molecular movement in cells
A.
_______________________________-
spontaneous, random
movement from high to low concentration
1.
Requires no energy input by cell
2. Remember that cell membranes are ____________________.
a. The cell membrane is only permeable to
_______________________________ molecules
and a
few
other __________________________
molecules like
oxygen (O2)
and carbon dioxide (CO2).
b. These diffuse freely in and out of the cell.
B. What are cell membranes not permeable
to?
1.
Charged molecules known as __________________________
There are 2 classes of ions…
a. __________________________ have a
positive charge.
This includes _______________________________
b. __________________________ have a
negative charge.
This includes _______________________________
2.
Small hydrophilic (water soluble) molecules like ____________.
3.
Macromolecules like proteins and RNA
C. So, how do these particles get past the cell
membrane barrier?
1.
Facilitated Diffusion-
a.
Transmembrane
proteins create a water-filled pore
through which ions and some small hydrophilic
molecules
can pass by diffusion.
b.
The
channels can be opened (or closed) according to the
needs of the cell.
c.
Because
we are moving along a concentration gradient,
this costs the cell ______________________________.
d. ____________________________ enters cells through
facilitated diffusion.
2. Active
Transport-
a.
Involves
special transmembrane proteins called
transporters.
b. __________________________
enter and leave cells
through active transport.
D.
The diffusion of water across a selectively permeable membrane is so
crucial & vital that it is given a
special name, ______________________.
II. Why is water such a special molecule?
A.
The
chemical formula for water is ___________________________.
Water
is a ______________________.
that is made up of two atoms of hydrogen
and one atom of oxygen.
1.
Atoms have a nucleus where you find __________________.
a. Each proton has a positive charge.
2. Circling around the
nucleus are ______________________.
a. Each electron has
a negative charge.
b. The electrons
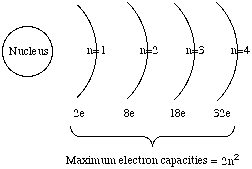
occupy electron shells.
c. The innermost
electron shell can hold 2 electrons.
d. The next electron
shell can hold 8 electrons.
3. Atoms and molecules try to be as electrically
stable as
possible.
Two ways they can achieve this stability include…
a. Remaining
electrically neutral (i.e. the number of
electrons must equal the number of neutrons)
1) If an atom or molecule has more electrons
than protons, it will become negative.
2)
If an atom or molecule has more protons
than electrons, it will become positive.
b. Filling their outermost electron shells.
B.
Water is a _____________________________________
molecule.
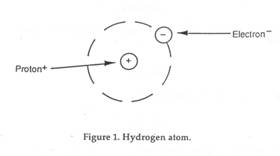
1. The atomic number of ____________________ is 1.
That
means
that it has one proton in its nucleus and 1 electron in its
electron
shell.
a.
To
fill that electron shell, it would have to gain an
electron.
b. That would make it less stable because it would have a -1
charge.
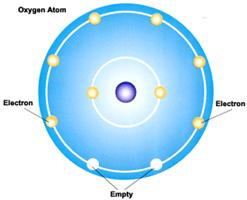
2.
The atomic number of ______________________
is 8. Oxygen
has 8 protons in its nucleus. It has 2 electrons in its innermost
electron shell and 6 electrons in the next electron shell.
a.
To
fill its outermost electron shell, it would have to gain 2
electrons.
b. That would give it a -2 charge, which is less stable.
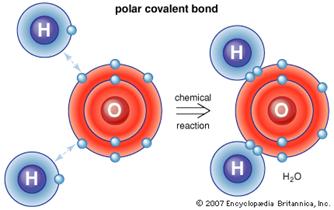
3. _____________________________________
are formed by
covalent
bonds. Two hydrogens
and one oxygen share their
electrons.
a.
This
allows the atoms to have their outermost shells filled
at least part of the time, increasing ___________________.
b.
By
sharing their electrons, they avoid gaining negative
charges.
C.
Water is a ______________________
molecule.
1. Oxygen has a +8 charge in its nucleus. The two hydrogen
atoms have only a +1 charge in their nuclei.
a. Because Oxygen has
a greater positive charge, it attracts
the electrons more strongly.
1) Think about a big kid on a playground by the
swings.
The big kid will get to swing more often and
longer.
b.
Thus,
oxygen develops a partial
__________________
charge (δ- ).
c.
The
hydrogen develops a partial
__________________
charge (δ+ ).
|
|
|
Electronic
Distribution in H2O |
2. Because it is both partially positive and partially negative, it is
attracted
to & dissolves anything that has
a charge.
a.
Water
is referred to as the ______________________.
b.
Because
it is both partially positive and
partially negative,
it is attracted to & dissolves anything that has a charge.
|
|
|
Table Salt Dissolving in Water |
3. Water molecules are also attracted to each
other, forming
hydrogen bonds, resulting in ______________________________.
|
|
|
Hydrogen Bonding between Water Molecules |
4.
As a general rule, ____________________________________
____________________________________________________.
Important topic: Why water moves in & out of cells
III. ______________________ – the diffusion
of water from an area of high
concentration,
across a semi-permeable membrane, to an area of low
concentration.
A.
Why does water move into and out of cells?
1. It depends upon the type of solution the cell
is in.
a. Water is a __________________. Anything dissolved in
water is a ______________________.
c.
As
the solute concentration in a solution _____________,
the water concentration ______________________ &
vice versa.
2. We are going to look at an imaginary plant
cell.
a. Our cell will be
75% water (we will use X’s to represent
water).
b. Our cell will be 25% solutes (we will use O’s to represent
the solutes).
|
|
3. First, let’s put our cell into a ____________________ solution –
one
that has a lower water concentration than the cell.
a. We will put the
cell into a solution that is 50% solutes and
50% water.
1) Water moves from higher to lower
concentration.
2) The cell is 75% water,
the solution is 50% water.
3) There will be a net movement of water out of
the
cell into the solution.
|
Hypertonic Solution |
The Results for the
Cell |
|
|
The cell died! Then the plant wilted and died! YIKES! Why didn’t someone water this plant? |
b. In hypertonic solutions, water will exit the cell.
1)
The cell’s plasma membrane will shrink away from
the cell wall.
2)
This is known as ______________________. The
cell will die.
c. Plant cells that
become dehydrated lose their shape.
This results in the plant ______________________.
4.
Next, let’s put our cell into an ______________________
solution – one that has the same concentration
of water as the
cell.
a. We will put the
cell into a solution that is 25% solutes and
75% water (just like the cell).
1) Water moves from higher to lower
concentration.
2) However, the cell is 75% water and the
solution is
75% water.
Therefore, there is no area of higher or
lower water concentration.
3)
There
will NOT be a net movement of water.
|
Isotonic Solution |
The Results for the
Cell |
|
|
This solution is perfect for both
plant and animal cells. |
b. In this solution,
the plasma membrane of the plant cell will
be pushed up against the cell wall.
1) This is actually due to water entering the
central
vacuole of the cell, pushing against the
cytoplasm.
2)
If the plant loses any water, it will wilt.
5. Finally, let’s put our cell in a ______________________ solution
– one
that has a higher water concentration than the cell.
a. We will put the cell into a solution that is 0% solutes and
100% water.
1) Water moves from higher to lower
concentration.
2) The cell is 75% water,
the solution is 100% water.
3) There will be a net movement of water into the
cell
from the solution.
|
Hypotonic Solution |
The Results for the
Cell |
|
|
The cell wall (through turgor
pressure) saved the cell! Hooray! (An animal cell will swell & burst.) |
b. In these
solutions, animals cells swell & burst.
c. However, plant
cells have a rigid cell wall that prevents
this.
d.
These
cells are said to be ______________________.
They
are filled with water. This plant will
not wilt. ***This
is the normal condition of plant
cells.***
IV. Osmosis in Plants
A.
Recall
that osmosis moves water from an area of high water
concentration to an area of low water
concentration. It requires
______________________.
1.
Plants have to constantly cope with changing osmotic pressures
due to changes in water availability and
transpiration rates.
B.
Unlike animal cells, plant cells don’t explode
in hypotonic solutions
due to turgor
pressure.
1.
Turgor pressure –
2.
Plant cells store ions, sugars, organic and amino acids and
other
substances in considerable concentrations in their
_______________________________.
a.
Remember
that wherever a charged particle is or goes,
____________________________ is bound to follow.
b.
Therefore,
the solutes in the central vacuole cause water
to rush into and fill the vacuole with
water.
c.
As
the vacuole fills with water, it presses against the
cell’s cytoplasm, which causes the plasma
membrane to
press against the ____________________________.
d.
This
pressure prevents more water from moving in by
osmosis.
3.
This causes plant cells to build up a large positive internal
pressure
(pushing against the cell wall), the __________________.
4. Turgor pressure has
a decisive influence on the maintenance of
the rigidity and stability of plant
tissues.
a.
Plants
that lose water ____________________________
because their turgor
pressure decreases.
C.
In plants, we talk about the potential
water molecules have to move.
This is the ____________________________.
(Think of this as the
concentration
of water.)
1. Distilled water has the greatest osmotic
potential of any
solution.
2. Adding anything to the water (sugar, salt,
etc.) lowers the
solution’s osmotic potential.
3. Water molecules always move from greater to
lesser osmotic
potential.
D. ____________________________– the
osmotic potential of a cell.
1. For animal cells, the water potential is the
osmotic potential of
the
cytoplasm.
2. In plant cells this is different.
3. Plant cells have a cell wall, which exerts an
inward pressure
when
the cell is turgid.
a.
This
is known as the pressure potential
(pressure
b.
potential = turgor
pressure).
4. The water potential of a plant cell is equal
to the osmotic
potential of the cytoplasm plus the cell wall
pressure, OR…
W.P. = O.P. + P.P.
E. THIS IS THE RULE OF WATER MOVEMENT IN
PLANTS:
1.
Water always moves from an
area of high water potential to an
area of low water potential.
Important topic: Why water moves in plants
V. Molecular Movement In Plants- So, water and solutes (anything
dissolved in
water to make a solution) are moving around
in plants. What types of
movements do we see & why?
A.
_______________________________- attraction
(adhesion) of water
molecules to colloidal substances –
1.
Water molecules are attracted to any charged particle
(_______________________________).
2.
The water molecules attract more water molecules
(_______________________________).
3.
Works in a manner similar to osmosis.
4.
This is how seeds expand when soaked in water.
B.
Transpiration-
1. Approximately _________________________ of the water that
a plant
absorbs is lost by transpiration!
2.
Transpiration is also partially responsible for water movement
through the xylem, and the prevention of heat
damage to plant
cells.
3.
Typical transpiration rates (L/day):
a. Mature corn
plant- 15
b.
c. Birch tree-
200-1000
C & D: Why water moves up the xylem
C. _______________________________________
– first discovered
& measured by
Stephen Hales.
***Botany’s first attempt to explain
why water moves from the ground up a
plant.***
1.
The
force that drives the water through the root is based on
differences in the water potential of the root's
surrounding
(usually soil) and its xylem sap.
a. The _________________ that have passed
through the
__________________ are usually trapped
and cannot leave
the ________________________
any more.
b. The built-up ions attract water by __________________.
c.
Thus, root pressure develops. The build-up of water
presses the water in the xylem (with its
dissolved ions, called
the __________________________________________)
upward.
2. When a tomato plant is carefully severed
close to the base of
the
stem, sap oozes from the stump. The fluid comes out under
pressure
due to root pressure.
3.
Although
root pressure plays a role in the transport of water in
the xylem in some plants and in some
seasons, it does not
account for most water transport.
a.
Root
pressure is always small, and doesn’t even develop
in some plants.
b.
The
highest root pressures occur in the ___________
when the xylem sap is highly ___________________ to
soil water, but the rate of transpiration
is low.
c.
In
the summer, when transpiration is high and water is
moving rapidly through the xylem, often no
root pressure
can be detected.
D. _______________________________- Water rises in small tubes
(tracheids and vessels) to some
extent because of adhesion to the
tube walls (capillarity) and cohesion
between water molecules.
***Botany’s BEST
explanation for why water moves up through
xylem.***
1.
___________________ - Spontaneous
movement of liquids up
or down
narrow tubes, or capillaries.
a.
The height that water will rise in a tube is ____________
proportional to the
diameter of the tube.
b.
The
larger the diameter of the tube, the smaller the
percentage of water molecules in direct contact
with the
glass and, correspondingly, the __________________
the rise in the water in the tube.
2.
Water
in the xylem can be thought of as columns of water
molecules held together by cohesion.
3.
As
water transpires from the leaves of a tree, it results in a lower
water potential in the leaves.
4.
More
water enters the roots by osmosis due to the subsequent
decrease in water potential in the xylem.
5.
Rapid
transpiration can cause the roots to grow rapidly towards
the nearest source of water.
6.
During rapid transpiration, water may move through the roots by
_____________________________—simple movement from high
water potential to low water potential—with
little osmosis taking
place.
E & F: Why water moves down the phloem (with food)
E.
_______________________________________________________-
Organic
solutes move from an area of high concentration
(_______________________________) to an area of low concentration
(_______________________________)
by diffusion.
1.
For
example, glucose (sugar) is produced in the leaves & then
stored in the roots of some plants. In this case…
a.
The
leaves are the source (there is a greater
concentration of sugar
in the leaves where it is being
produced).
b.
The
roots are the sink
F. _______________________________
***Botany’s
BEST explanation for why water moves down through
phloem.***
1.
At
the source (the photosynthesizing cells in the leaves), sugar
is actively transported into phloem
cells.
2.
Sugar
is a solute. Because the sugar (or solute)
load in the
phloem cells has increased, the
concentration of water in these
cells decreases.
3.
Water
flows by osmosis from an area of higher concentration
into these phloem cells.
a. This osmosis creates ____________________________
________________________________________________
which drives the fluid through the
sieve-tube cells.
4.
At
the sink (any cell that needs sugar to carry out its functions),
sugar is actively transported out of the
phloem
a. Again, water again follows by osmosis, decreasing the
turgor pressure near the sink.
5.
Phloem
fluid moves by mass flow from high turgor pressure
(the
leaves or source) to low turgor
pressure (any cell needing sugar
or sink).
6. As sugar is used up by the cells, its
concentration decreases.
a. As the solute (sugar) concentration decreases, the water
concentration increases.
b.
Again,
water moves from high to low concentration, so it
now leaves the cells.
c.
This
water moves into the ________________________
where it is recirculated
or transpired.
III. Regulation of Transpiration- a plant MUST maintain a balance
between
_______________________________ (necessary to make sugar) and
_______________________________ (through transpiration) by opening
and
closing its __________________________. (Think
about the dangers of the hot
summer sun
– plant wilting! YUCK!)
A. Stomata- openings in the epidermis formed by two guard cells.
1.
They are responsible for the regulation of transpiration and gas
exchange.
2.
The
inside wall of each guard cell is thicker than the outside
wall.
3.
When turgor pressure increases (due to water
entering the cell’s
vacuole), the outside wall expands more than
the inside wall.
4.
This causes the inside wall to bow out and open the stoma.
5.
Stomata open when the guard cells actively transport
_______________________________
into their cytoplasm.
a. Water follows by osmosis and turgor
pressure increases.
6. Under water stress, the water potential
outside the cell becomes
lower than inside the cell.
a. Water leaves the cell by osmosis and the stomata close.
7. The hormone _______________________________may be
produced during water stress and cause the
guard cell membranes
to leak potassium, thus decreasing the turgor pressure.
A.
_______________________________- most plants have their stomata
open during the day so that CO2
can enter for photosynthesis. Some
plants (CAM) found in arid regions only open
their stomata at night in
order to decrease water loss. They have a
specialized form of
photosynthesis.
C. Transpiration Rate Factors:
1. Light- due to
photosynthesis. __________________________
2. CO2
concentration- due to photosynthesis
3. Air Currents- cause
gradients in water vapor over the stomata.
_______________________________
4. Humidity- inversely
correlated with transpiration rate.
_______________________________
5. Temperature- positively
correlated with transpiration rate.
_______________________________
D. _______________________________- production of water droplets
on leaves of some plants by structures
called ___________________.
1.
Usually occurs when a cool night follows a humid day.
2.
As the water evaporates during the day, solutes are left behind.
3. At night, these solutes attract water, which
beads up on the
leaves’ margins.
VII. Plant nutritional requirements- most plants get their mineral from the
soil
and soil water.
A. Minerals are non-organic nutrients that are
necessary for proper plant
metabolism.
1.
Organic elements- constitute most of the dry weight of the plant.
a. Includes C, H, & O
2.
Macronutrients- used in large amounts by plants, and constitute
0.5-3% of dry
their weight.
a. Includes N, K, Ca, Mg, & S
3.
Micronutrients- needed in small amounts, and constitute only a
few ppm of plant dry weight.
b. Includes P, Fe,
Na, Cl, Cu, Mn, Co, Zn, Mo,
& B
4. When
necessary, elements are deficient in the soil, the plant
will not
grow normally.
a.
Fertilizers
are used to replace or increase nutrients in the
soil.
B.
Uses of key elements in plants:
1.
N- proteins, nucleic acids, chlorophyll
2.
K- activates enzymes, concentrates in meristems,
controls guard
cells
3.
Ca- middle lamella, membrane transport
4. P- nucleic acids, coenzymes, activates
enzymes, cell division
5.
Mg- activates enzymes, chlorophyll
6.
S- some amino acids
7.
Fe- chlorophyll, respiration
8.
Mn- activates enzymes
9.
B- influences use of Ca, but overall function unknown










